Your Bioanalytical Services Powerhouse
Bioassay, a Reaction Biology Corporation, offers GMP potency assays and functional bioassays, both in vitro and in vivo, as well as GLP compliant services. Through our sister company PSL, Peptide Synthesis Services, we can also provide peptide-specific antibody production and neoantigen synthesis.
We are a trusted provider of development, validation and routine bioanalytical assays for global players and biotech companies in the pharmaceutical, chemical and cosmetic industries.
We develop and perform GMP potency assays for a wide variety of drugs and biologics, including proteins, monoclonal antibodies, vaccines and gene therapies. Our team of experts works with clients to select the most appropriate assay format and design a robust, QC-friendly and sensitive method. We follow GMP and ICH guidelines to validate potency assays, and offer routine GMP potency QC testing to support upscaling and manufacturing validation processes, as well as batch release and stability testing to monitor product potency over time.
Meet Our Team

Joanne McCorriston

Mareike Hoffmann
Learn More From Bioassay
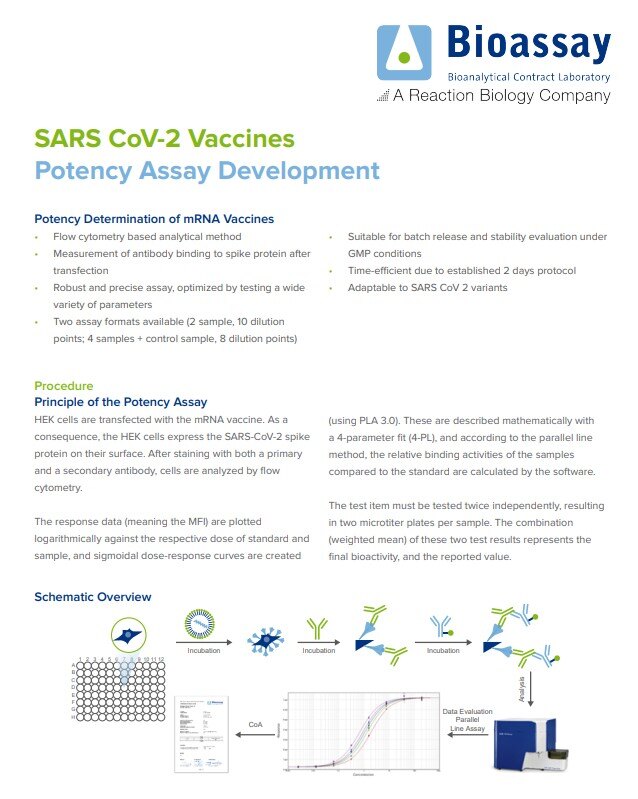
SARS CoV-2 Vaccines Potency Assay Development

GLP-1 Receptor Agonists Potency Assay Development

Advancing Solutions in GLP-1R Agonist & Antagonist Development

Validated In Vivo Tumor Models
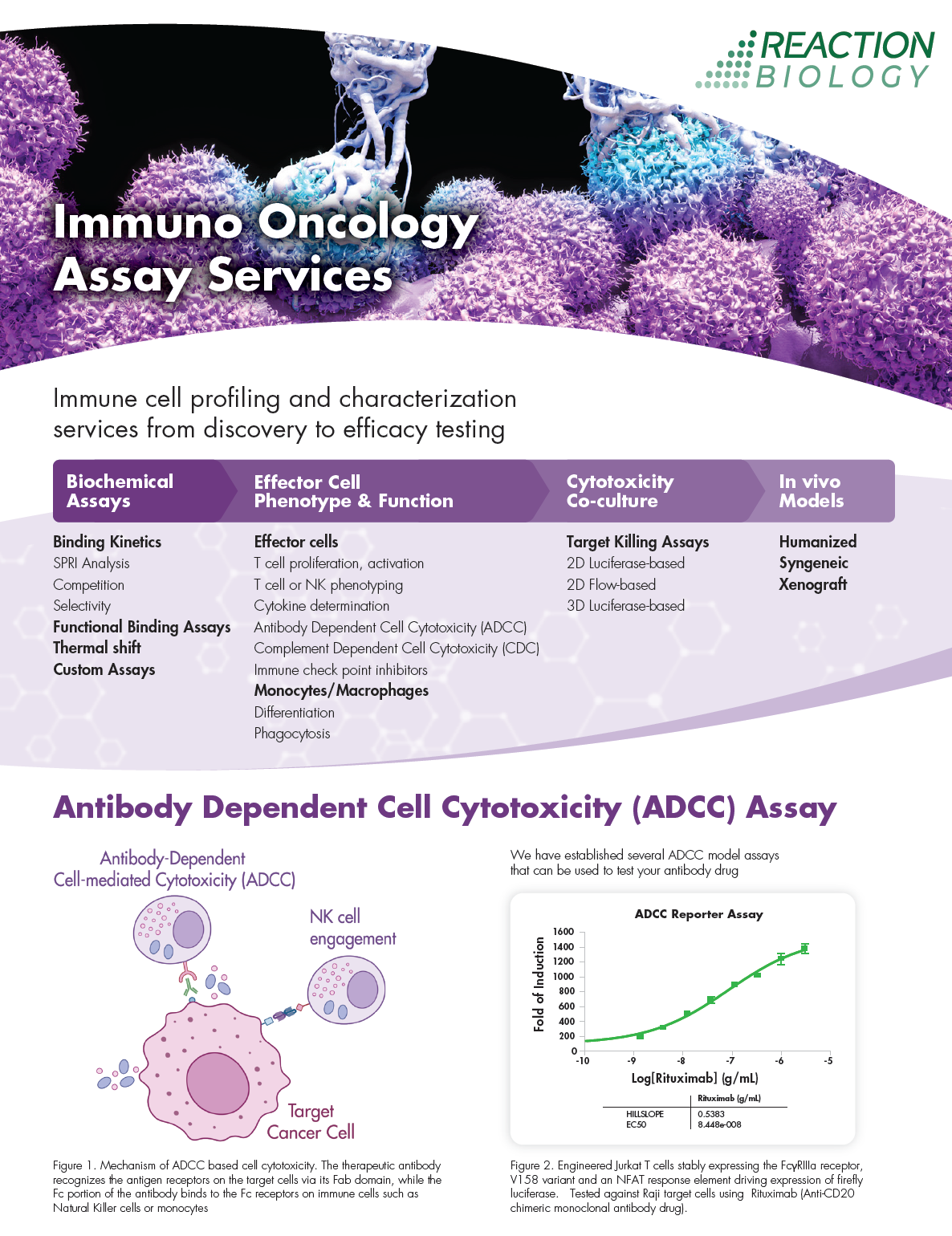
Immuno Oncology Assay Services
Macrophage Cell-Based Assays

White Paper: The Hollow Fiber Model

White Paper: SubQperior - The next generation of tumor models


